The catalysts for a major biotech rally in 2017 are already multiplying—not least because of the Trump presidential victory—and one little-known company is poised for huge potential gains as it prepares to unveil a breakthrough medical device that could save the U.S. government tens of billions of dollars a year, and help prevent the 15 million strokes suffered annually around the world.
Some six million people die from stroke every year, while another 5 million people are rendered permanently disabled. In the U.S. alone, nearly 800,000 people suffer from strokes annually—and someone dies of a stroke every 4 minutes.
If ever there was a market for anything, it is tools to aid in early stroke detection, and this is exactly what CVR Medical (TSX:CVM.V ; OTC:CRRVF) has been quietly and painstakingly working on for 10 years—and now it’s ready to charge out of the gate with its new, uniquely affordable technology.
A Lucrative New Era for Biotech
While Hillary Clinton had threatened the future of biotech companies by promising to go on the offensive against them, the unexpected victory of Donald Trump has analysts heralding a very bright and very lucrative new era for biotech. Speaking to Forbes, Todd Hagopian, who manages two mutual funds through Marketocracy.com, noted that with the Republicans in control of the White House and Congress, biotech companies are set up for an extraordinary comeback.
This is where the new barons are being made, with medical breakthroughs turning countless billions in new profits—and 2017 is set to be the strongest year ever.
Biotech companies are going to forge lucrative new frontiers this year, from cellular immunotherapies and liquid biopsies, to bioabsorbable stents and CVR’s Carotid Stenotic Scan (CSS), which detects stenosis within the Carotid Arteries—a serious risk factor in stroke.
We are also seeing major gains this year by cellular immunotherapies reporting 90 percent remission rates for acute lymphoblastic leukemia (ALL). This should go before the FDA this year and could trigger a wave of approvals for other blood cancers and lymphomas, and could eventually replace chemotherapy. Among many other positive developments in recent years, in December 2014, Amegen (NYSE:AMGN) won FDA approval for immunotherapy Blincyto, and large-cap AstraZeneca (NYSE:AZN) is also poised for major gains in redefining cancer treatment.
Liquid biopsies, or blood tests that uncover signs of actual DNA or cell-free circulating tumor DNA (ctDNA), could prove to be a revolutionary cancer test with annual sales forecast to be $10 billion. Cancer Genetics, Inc. (NASDAQ:CGIX) now has five programs to develop and validate multi-market liquid biopsy tests. And bioabsorbable stents, approved in the U.S. in July for the first time, have an amazing market potential approaching $2 billion in six years, with this year the critical juncture. Prominent North American players in this market include Abbott (NYSE:ABT) and Reva Medical Inc. (ASX:RVA).
It’s the perfect time for a small-cap company of top-notch professionals like CVR to release a new medical device that everyone is desperate for—from medical professionals, to insurance providers, to the billions of people who suffer a stroke every year.
3 Reasons to Keep a Close Eye on CVR Medical in the coming months:
1) The Market is Desperate for Affordable Stroke Solutions
When every second counts for a stroke sufferer, and current diagnostics take as long as three hours, CVR’s breakthrough Carotid Stenotic Scan (CSS) could potentially make the difference between life and death for millions because Carotid Artery Disease is the leading indicator of stroke.
It only takes on average 2 minutes, and the price tag on the device makes it affordable for providers and payers.
With strokes coming in as the second leading cause of death globally for people over the age of 60, and the fifth leading cause of death for people aged 15-59, a new technology that can deliver early detection of the leading indicator of stroke is beyond critical.
According to the CDC, early action is urgent for survival, and only 38 percent of stroke sufferers even recognize they are having a stroke in time to receive effective emergency intervention.
When you further consider that someone dies from a stroke every 4 minutes, CVR’s technology could end up being the most explosive thing on the medical scene in decades.
Let’s put this into perspective: In the same way that 3D seismic imagery revolutionized the oil and gas industry, letting explorers and producers find the sweet spots to drill in, CVR’s CSS technology plans to revolutionize stroke detection.
Until now, there has been no cost-effective way to screen for Carotid Artery Stenosis, which leads to Ischemic strokes—the most common type of stroke, which represent over 80 percent of strokes and cost the U.S. government alone tens of billions of dollars a year.
2) Affordable Answer to a Multi-Billion-Dollar Problem
CVR Medical’s CSS detects stenosis within the Carotid Arteries, potentially offering patients and caregivers a device for early detection in a quick and repeatable manner. Unlike other comparative modalities, the CSS was designed to function without the assistance of a certified technician. These three facts combine to create one of the potentially biggest—and most lucrative--phenomena in recent medical equipment market history.
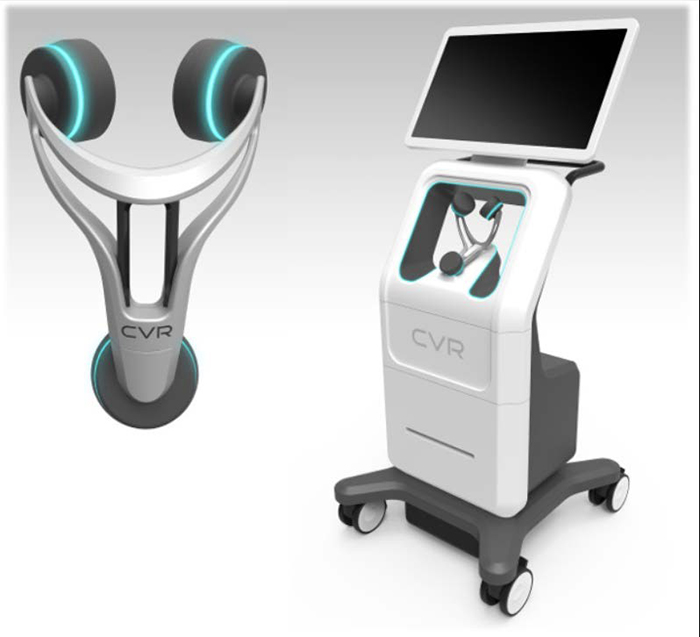
CVR invested $23 million in its breakthrough technology, with early stage clinical trials complete and now headed toward pivotal trials, eyeing the potential for immediate profitability, with possible U.S. sales alone up to 400,000 devices annually at $49,000 each. When you talk globally, the numbers are staggering.
The CSS makes a connection between fluid flow and sub-sonic frequencies to detect arterial disease or blockage. Blood flowing through the carotid arteries produces wave patterns which are shaped and altered by the presence of irregularities on the inner artery walls. CVR’s advanced technology captures these wave patterns and analyzes them mathematically with patented algorithms. After a brief test, the analysis is complete, offering a way to potentially identify those at risk of a stroke and arming the healthcare provider with the information necessary to prevent the deadly event.
Coming in at $49,000 per unit, compared to up to $2.5 million for existing technology.
Additionally, some existing technologies are invasive and, due to cost, not accessible to many. CVR’s CSS is positioned to potentially fill this current gap. Once available, the CSS technology stands to save the U.S. government up to $34 billion a year, the current cost of strokes annually in the U.S. according to the CDC.
For investors, medical providers, insurers, and the public at large, the secondary benefit is that this new innovation has a price tag that renders testing up to 50 times cheaper than many tools on the market today.
3) The Final Phase Before Breakout
We are now right at the end-game here for this medical breakthrough, and the market impact could be quite dramatic.
After investing $23 million into R&D and keeping quiet about their findings and results for 10 years—CVR is at a critical junction where investment could soon to into significant profit. The company is just coming out of the early stage of clinical trials and is about to enter pivotal trials—and this is where the window of opportunity lies for investors.
What everyone should be keeping a close eye on right now is the release of the preliminary clinical report very soon, and then the full clinical report roughly 4-8 weeks later. Once they have this, it goes to the FDA to prove safety and efficacy and then market clearance and delivery.
And CVR is confident about the final round of clinical trials and FDA approval.
Where the big picture gets even better is the guaranteed market, and the costs. CVR’s all-in costs are less than half the sales costs—so the company is expecting high profitability right out of the gate, and it’s got manufacturing lined up already, just waiting for the final hurdles to be cleared.
The market potential here is some 235,000 medical offices—just in the U.S., and just for starters. If you do that math at just one device per office, the revenue is potentially staggering. But what investors really like to hear is that it won’t stop here: CVR’s breakthrough early detection stroke technology is only the beginning of series of breakthrough medical devices this company has in the works.
The era of biotech is here, and by all accounts, the political environment will welcome it with open arms, and the market has never been hungrier. At the center of it all, we have CVR—and hopefully very soon, an answer to a needless death every four minutes.
Legal Disclaimer/Disclosure: This piece is an advertorial and has been paid for. This document is not and should not be construed as an offer to sell or the solicitation of an offer to purchase or subscribe for any investment. No information in this Report should be construed as individualized investment advice. A licensed financial advisor should be consulted prior to making any investment decision. We make no guarantee, representation or warranty and accept no responsibility or liability as to its accuracy or completeness. Expressions of opinion are subject to change without notice.We assume no warranty, liability or guarantee for the current relevance, correctness or completeness of any information provided within this Report and will not be held liable for the consequence of reliance upon any opinion or statement contained herein or any omission. Furthermore, we assume no liability for any direct or indirect loss or damage or, in particular, for lost profit, which you may incur as a result of the use and existence of the information, provided within this Report.